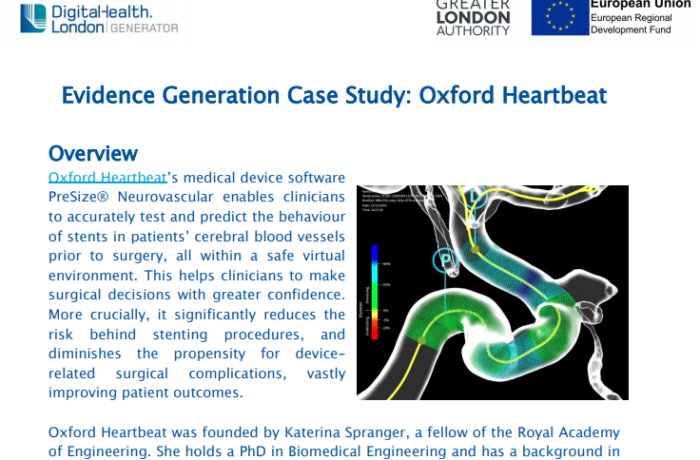
NHS keeps its finger on the pulse with rollout of innovative KardiaMobile to accelerate diagnosis of potentially fatal heart rhythm condition
Posted on
London, UK, 15 February 2018: KardiaMobile, a revolutionary and potentially life-saving heart monitor is being distributed to the NHS in England. The device, about the size of a credit card, detects atrial fibrillation. AF is the most common arrhythmia (irregular heart rhythm disorder) and a contributing factor of up to 1 in 5 strokes in the UK.1 KardiaMobile, developed by AliveCor, is being rolled out as part of an NHS England-funded project, via the country’s Academic Health Science Networks (AHSNs). To date, the AHSNs have supported the uptake and spread of Kardia Mobile through the NHS Innovation Accelerator (NIA).
AF affects 1.4 million people in the UK, however it is estimated that almost a further half a million people are undiagnosed and at increased risk from potentially fatal AF-related stroke.2 Patients with AF often remain undiagnosed because their symptoms come and go and may not be present during a consultation with their GP. This means those with suspected AF are sent to hospital for costly ECGs, and that it may take several visits, and several months or even years before they are diagnosed.
Using a compatible smartphone or tablet device, KardiaMobile is able to record the electrical activity of the heart through a person’s fingertips. The connected app delivers an accurate electrocardiogram (ECG) reading to a device in less than 30 seconds and will indicate whether a person has possible AF.3 The app allows heart rhythm recordings to be viewed, saved and shared with healthcare professionals (HCP) allowing for faster detection and diagnosis of AF.
“Introducing this innovative technology to GP surgeries and hospitals gives healthcare professionals a quick and simple way of recording an ECG without the need to send a patient to hospital. Due to the size of KardiaMobile patients can even take the device away from a consultation. Patients can record their heart’s electrical activity whenever. The device can be used at any time, regardless if they show signs of symptoms or not. This ultimately means AF can be diagnosed faster, anticoagulation therapy can be prescribed to reduce the risk of an AF-related stroke and treatment for AF can be accelerated,” said Trudie Lobban MBE, Founder & CEO at Arrhythmia Alliance & AF Association.
This simple-to-use technology will also offer real cost savings to NHS. By reducing costs needed to achieve an AF diagnosis, KardiaMobile could save the NHS an estimated £2 billion.4 This does not even take into account the longer-term benefits and financial savings of potentially preventing severe and costly AF-related strokes.
The NIA is delivered in partnership with England’s 15 AHSNs. Professor Gary Ford, Stroke Physician and lead on the project for the AHSNs said: “More than 420,000 people throughout England are unaware they have irregular heartbeats and of the dangers that this can pose to their health. We have highly effective treatments that can prevent these strokes, but early detection is key. Using cost-effective technology, the NHS will now be able to identify people with irregular heartbeats quickly and easily. This will save lives.”
“As the NHS approaches its 70th birthday this year, this is also a great reminder of the way that healthcare is continually evolving and innovating. Taking advantage of digital health solutions will be even more important for the next 70 years. Today’s new devices are just one example of the way that low-cost tech has the potential to make a huge difference.”
Francis White, Vice President of AliveCor, commented: “We are delighted to have the opportunity to partner with the NHS. Providing our technology to GPs and hospitals around the UK offers a more efficient solution to AF diagnosis and may ultimately improve patient outcomes.”
KardiaMobile is also available for individuals to purchase on Amazon, alongside AliveCor’s latest product; KardiaBand which integrates with the Apple Watch. Both devices allow patients to monitor their own heart rhythm and record ECG readings for analysis by healthcare professionals.
About KardiaMobile
To record an ECG, patients place one or more fingers from the left hand onto one of the Kardia sensors and one or more fingers from the right hand onto the other sensor. A clinically equivalent ECG reading is then taken for 30 seconds. The Kardia app analyses and displays the ECG trace classifying the reading as “Possible AF”, “Normal” or “Unclassified” (outside of normal definition but not AF). The reading can then be shared with a HCP to confirm diagnosis and discuss appropriate treatment.
KardiaMobile has been CE‑marked to AliveCor as a Class IIa medical device and approved by the FDA in the USA for the detection of AF. It is one of the most clinically validated mobile ECG solutions available and is recommended by leading cardiologists as well as being used globally for accurate ECG recordings. Clinical studies have demonstrated that Kardia delivers diagnostic quality ECG recordings and its accuracy is comparable to readings from lead 1 of standard ECG machines.
KardiaMobile was selected for the first-year cohort of the NHS Innovation Accelerator (NIA) – a national initiative that’s improving people’s lives through supporting the spread of high impact, evidence-based innovations across the NHS. For more information visit www.nhsaccelerator.com
About AF
AF is an abnormality in the hearts’ natural rhythm, also known as an arrhythmia. It is caused by the upper chambers of the heart (the atria) producing abnormal electrical impulses. These cause the atria to quiver or twitch, known as fibrillation, and prevents the heart from beating effectively.
AF can increase the risk of stroke. The arrhythmia causes blood to pool and this can cause a blood clot to form. If the blood clot is carried to the small blood vessels in the brain it can block blood flow to the brain resulting in a stroke.
More information can be found at Arrhythmia Alliance.
About AliveCor
AliveCor, Inc. is pioneering the creation of FDA-cleared ‘machine learning’ techniques to enable proactive heart care and is now recognised around the world for transforming cardiac care. AliveCor was recognized as a 2015 Tech Pioneer by the World Economic Forum and one of the 50 Smartest Companies in 2015 by the MIT Technology Review (#14). AliveCor is a privately-held company headquartered in Mountain View, Calif. For more information, please visit www.alivecor.com and follow @AliveCorUK #KardiaMobile #knowyourpulse